North Bristol NHS Trust (NBT) is one of eight National Institute for Health Research (NIHR) supported sites across the UK where a new coronavirus vaccine trial - referred to as the COVID-19 Heterologous Prime Boost study, or ‘Com-Cov’ - is taking place. The trial is backed by £7 million of government funding and will recruit over 800 volunteers and among those taking part is H M Lord-Lieutenant for Bristol Mrs Peaches Golding OBE.
The aim of the vaccine trial is to determine what happens when two different vaccines are given to the same patient, in addition to looking at the differences between 4 week and 12 week intervals between doses. Patients do not know whether they will receive the Oxford vaccine manufactured by AstraZeneca first or the Pfizer BioNtech vaccine or whether they will receive the same vaccine during both injections. However, the results will have global implications.
‘I have absolute faith in the safety of the vaccines being studied and in the professionalism of the team carrying out the trial here at Southmead,’ explains Peaches Golding. ‘If the findings of this study can increase the flexibility with which the vaccines are deployed in the UK and abroad, then I will feel that I have made a positive contribution to the enormously successful research effort taking place in the UK.’
In the UK, over 120,000 people have died within 28 days of being diagnosed with COVID-19.
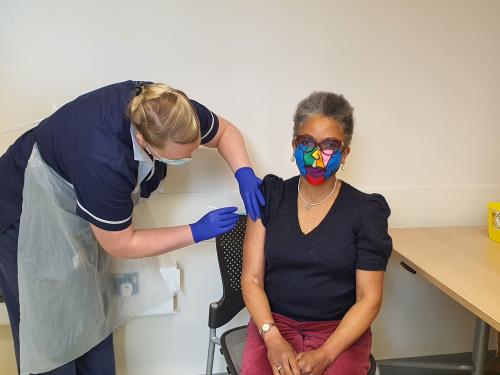
Mrs Golding’s first jab took place on Saturday 20 February at the Learning and Research Building at Southmead Hospital.
Watch this video of Peaches telling us why she was so passionate about taking part in a COVID-19 vaccine trial and why she is encouraging people from a Black, Asian and Minority Ethnic background to get the COVID-19 vaccine when they are invited to do so.
