Information Sheet 2: Taking and Sending Pregnancy Loss and Live Patient Samples for Genetic Testing
Tel: 0117 414 6150
E-Mail: swglhprenatal@nbt.nhs.uk
Suitable samples:
Suitable samples for genetic testing of pregnancy loss are biopsies of skin, cord or placenta. Samples may be refrigerated (4oC) overnight. DO NOT freeze any samples.
NOTE: A sample of fresh placental tissue may be sent in addition to/instead of fetal tissue.
Container:
All samples should be put in a sterile container, such as a plastic universal containing tissue biopsy medium, if available, or sterile normal saline.
Plastic universals containing skin biopsy medium are available, on request, from the laboratory. If stored frozen (-20oC) the media has a shelf life of 3 months. Media is likely to arrive defrosted, this is not an issue so please do not discard it. Please re-freeze until day of use. Please ensure tissue biopsy medium is completely defrosted prior to use and that the media is clear when held up to the light. Non-sterile containers and those contaminated with formalin or formal saline are not suitable. Please do not send pregnancy loss samples in CVS media, the skin biopsy media has additional reagents that will protect the pregnancy loss samples from a wider range of infections.
Transport:
Send by post or hospital transport to the address below, including an appropriately completed referral form which can be downloaded on the Bristol Genetics Laboratory (BGL) page.
Bristol Genetics Laboratory, Severn Pathology, Southmead Hospital, Westbury-on-Trym, Bristol, BS10 5NB
In the event of anticipated postal delay, (e.g. bank holidays) samples may be sent by courier, as long as arrival is within working hours. Please inform the laboratory of the expected time of arrival. The majority of samples received by the laboratory within 3 days of being taken produce a result.
Skin biopsy from fetus/stillbirth
- The biopsy is usually taken from the upper outer thigh within the area normally covered by the nappy
- Rinse the limbs thoroughly with sterile normal saline
- Using sterile instruments, cut an ellipse of skin 1-1.5cm in length (Fig. 1) to a depth which includes the full thickness of the skin and a small amount of underlying subcutaneous fat, and place in a sterile container of skin biopsy medium or sterile saline.
- For samples requiring fibroblast culture for storage or metabolic testing please send the sample in skin biopsy medium and as soon as possible after death to increase the likelihood of cell growth in culture
The ellipse should leave the fetus with minimal damage which can be easily repaired by clinicians or at post mortem (Fig. 2)
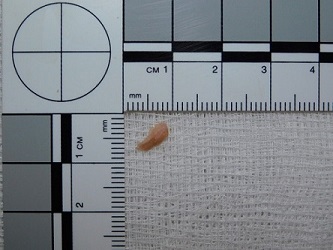
Fig 1: An ellipse of skin taken at Post Mortem for genetic testing (please do not take smaller biopsies than the size shown.
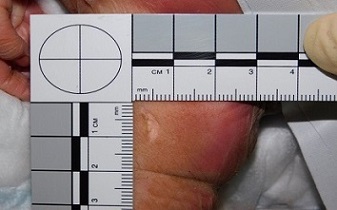
Fig 2: Site of biopsy after removal of sample sent for genetic testing.
Skin biopsy from live patient:
- The biopsy is usually taken from the inside of forearms, the upper thigh or (in babies) the iliac crest. A local anaesthetic may be used if required.
- Clean the skin surface with ether/alcohol and allow to dry
- Hold the piece of skin in fine forceps, placed horizontally to skin surface so a hump of skin 5-10mm long, 1-2mm wide and 1-2mm high projects above the edge of the shafts.
- Exert gentle pressure for 30 seconds, until the skin becomes pale and translucent.
- Remove the protruding skin with a sterile scalpel. Place in skin biopsy medium.
- These samples should be sent by hospital transport or first class post as delays reduce the chance of culture success
- Please use skin biopsy medium to increase the chance of culture success.
NOTE: The biopsy should be deep enough to include at some point the whole thickness of the epidermis and adjacent connective tissue.

Fig. 3: A section of cord sent for genetic testing (approximate length 2cm, diameter 1cm)
Cord biopsy from fetus/stillbirth:
Cord samples are a good alternative to skin and can be taken without damaging the fetus. Cord samples can be used for routine genetic testing, although DNA quantity may be low. If possible please send a cross section of the cord (Fig. 3) of at least 2cm length (longer if the cord is from a loss at an early gestation as it is the total amount of material available that is important). Please send following the instructions outlined in the skin biopsy section.
Placental biopsy from fetus/stillbirth:
Fresh placental tissue is suitable for DNA extraction and testing, or cell culture instead of/in addition to fetal tissue where available (Fig’s 4 & 5). Please send a sample of chorionic villi as shown in the image below. Please do not send:
• large ERPCs
• Whole fetuses unless from a very early gestation (first trimester only) as we do not have the equipment or resources to handle them
Large ERPCs or whole fetuses from a later gestation will be returned unprocessed for an appropriate sample to be obtained and sent for testing. If the resources to take an appropriate sample are not available to the referring department, we recommend contacting a pathology laboratory at the referring Hospital for a biopsy to be taken and forwarded to BGL.
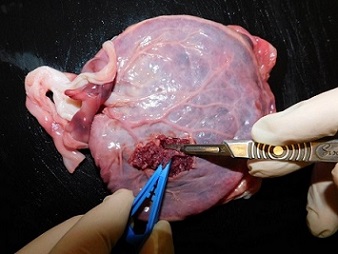
Fig. 4: Whole placenta with cord attached. Image shows placental villous biopsy being taken from beneath the fetal surface
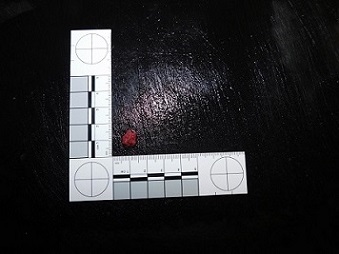
Fig 5: Placental biopsy taken for genetic testing (please do not send smaller biopsies than the one illustrated).
Cultured cell storage and DNA extraction:
DNA extraction and storage is carried out for all samples of an appropriate size. Cultured cell storage is carried out if specifically requested, for samples from live patients and sudden infant death samples. Informed consent must be obtained from patient/parents for storage of DNA or cultured cells.
Please note that the responsibility for gaining informed consent for testing and/or storage of material lies with the referring clinician. Upon receipt of samples the laboratory assumes that this consent has been obtained.
Disposal of samples
Excess skin and foetal tissue will be sent for witnessed disposal. Placenta, cord and membrane will be sent for routine clinical disposal.
Bristol Genetics Laboratory | Owner: Helena Hennessey | Approver: Catherine Delmege
Title: Information 2 - Procedure for taking solid tissue specimens.
Active Date Of This Version: 14/10/20223 | Version 21
